Author(s): Elias Chatzitheodoridis, Christos Georgiou
Editorial: Sohan Jheeta
Planetary and asteroid surfaces are irradiated by both particle and UV radiation from the Sun, as well as cosmic rays. Micrometeorites continuously induce fine fracturing of minerals. Both processes induce the formation of Reactive Oxygen Species (ROS).
ROS are metal superoxides in the form of O2•–, such as KO2 and NaO2, peroxides in the form of O22−, like Na2O2, K2O2, MgO2, and CaO2. All release H2O2 upon H2O-wetting. Hydroxyl (•OH) are classed as free radicals that can be contained in the planetary soils or regoliths.
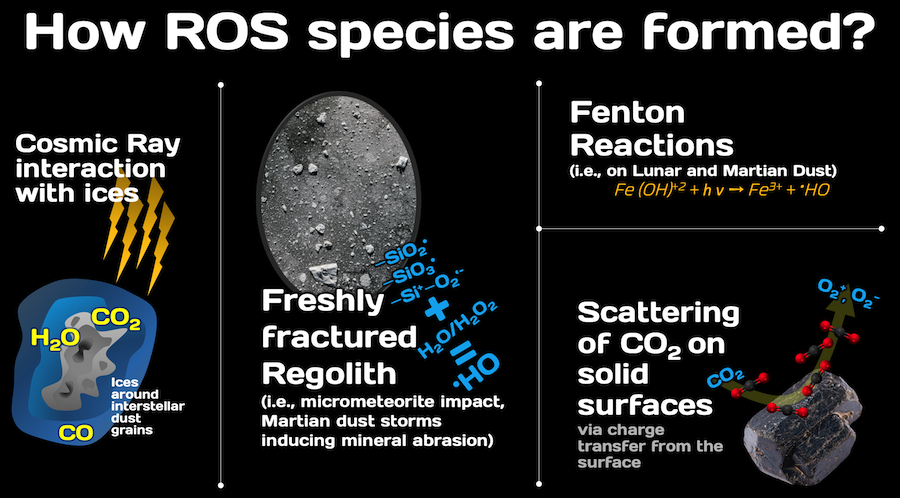
On Earth, ROS production in soils is typically associated with the relatively high abundance of O2 in the atmosphere. However, in other solar system environments, or space environments beyond our solar system, where gaseous O2 exists only in trace amounts (e.g., on Mars, Earth’s Moon, Europa, Saturn’s rings, and interstellar clouds), or even in planetary environments lacking gaseous O2, still ROS can be produced by many well-known natural processes.
For example, complex interactions between Saturn and its two satellites Titan and Enceladus; these satellites can generate oxygen and be transported to Saturn due to its high gravity. Ice water from Enceladus south polar plumes can be radiolytically oxidised to H2O2 and O2, by energetic particles from Saturn’s radiation belts (mostly electrons). Such ROS emanating from this radiolytic gas-driven cryovolcanism can be continuously accumulated deep in the icy regolith. Concurrently, H2O molecules escaping from Enceladus’ plumes should be split by magnetospheric plasma (protons, H+2, water group ions) into neutral and charged particles (O+), which can enter Titan’s atmosphere and be captured by fullerenes (hollow carbon atom shell, e.g., of C60). Exogenic keV O+ ions could become free oxygen within those fullerene aerosols, and eventually fall free onto Titan’s surface. Such processes could be driven by cosmic ray interactions with aerosols at all altitudes and can eventually drive pre-biotic chemistry.
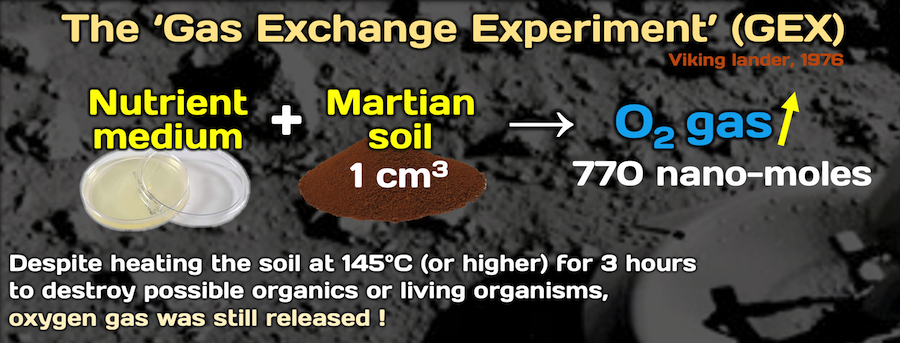
The observation that a Viking Mars mission biological experiment released oxygen gas fully justifies the development of a sensor instrument to detect ROS. In 1976, the Viking Lander performed biological experiments designed to detect extant life in the Martian regolith. The reactivity of the Martian regolith was first indicated by the release of O2 in the Gas Exchange Experiment (GEX), and the decomposition of organics, contained in a culture media used in the Labelled Release (LR) experiment. In the GEX, up to ~770 nmoles O2 was produced from 1 cm-3 regolith sample upon humidification or wetting. The persistence of O2 released from samples that were heated to 145°C for 3 hrs and then cooled, prior to wetting or humidification, ruled out a biological explanation of the GEX results.
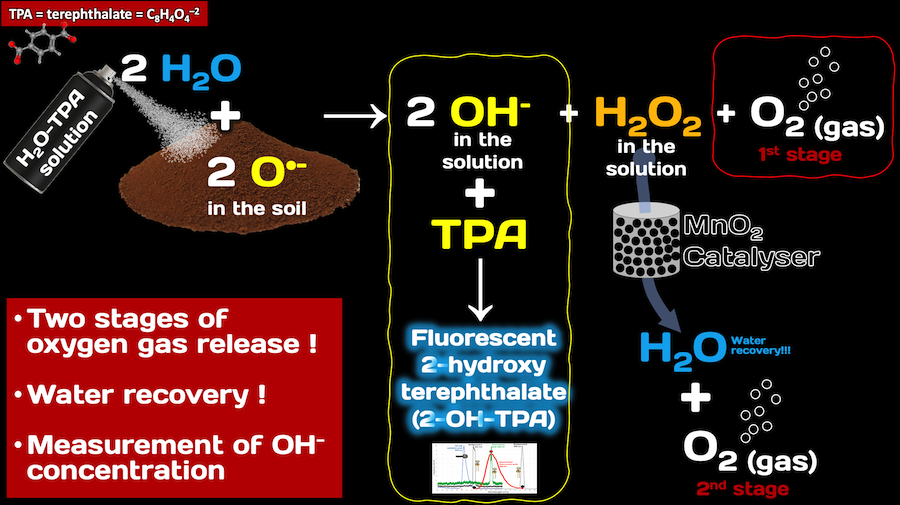
The OxR device is a novel device, designed to be of small footprint and fully automated, which measures ROS in planetary soils and regoliths. It is a temperature- and pressure-controlled system that is based on microfluidic technologies, with reagent reconstitution and delivery parts. The OxR instrument is also designed so that it can be used to identify ROS on other planetary and icy worlds, such as, Titan, Europa, Enceladus.
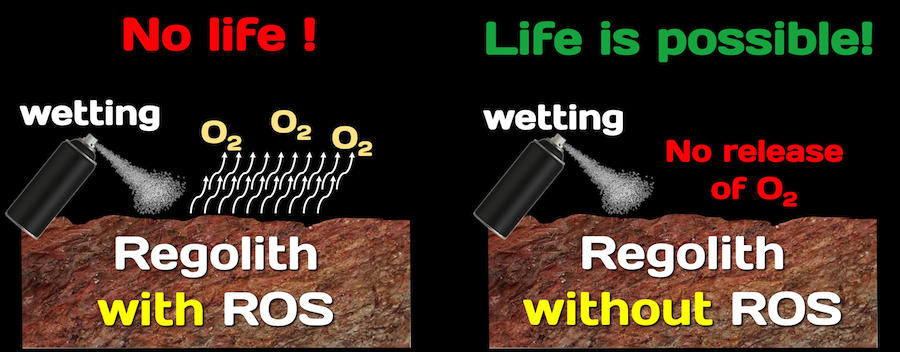
Considering habitation of Mars, the use of ROS will serve to:
- Identify soils that may contain preserved biosignatures, i.e., no ROS signals soils that can contain putative organics and life, presence of ROS indicates that even if organics or life existed, these should have been already destroyed.
- Identify soils for crop cultivation or to select areas where humans can settle down.
- In spaceships it can monitor toxic ROS development that is induced by cosmic radiation on walls, or in food and water storage tanks.
- Terrestrial applications include agriculture, especially on arid or overcultivated areas, or those affected by climate change.
- ROS is very well known to exist in mining areas, since it is naturally produced on the surfaces of some minerals; it is toxic to employees and therefore it should be monitored.



